Retroviruses: Poorly Understood Agents of Change
09/09/2017 / By News Editors

Investigating the alarming rise of chronic inflammatory diseases
Chronic inflammatory diseases have been skyrocketing in incidence in the past quarter century. The details explaining how retroviruses in today’s biological therapeutics including vaccines are contributing to autoimmune, neuroimmune disease and cancer are complex. Although I’ve spent my adult lifetime studying how retroviruses contribute to these diseases, paring down the complexities into basics is a daunting task.
(Article by Judy A. Mikovits, PhD republished from WorldMercuryProject.org)
In our book, Plague, Kent Heckenlively and I detailed the science and cover up surrounding my team’s 2009 discovery of a new family of human retroviruses related to mouse leukemia viruses, associated not only with cancer but with Autism Spectrum Disorders and Chronic Fatigue Syndrome. In Plague, my coauthor and I detail the science behind the discovery. Scientific research is not simply a study set in a defined space or time, but a lifetime of detailed observation and learning—a lifetime of forming hypotheses and modifying those hypotheses as technology and learning inform new discovery. Science is never settled as we learn each day and discover things that were once considered impossible.
However, science in the 21st century is more complex than ever in human history. Kent Heckenlively is a sixth-grade science teacher. In order to tell my story in a way everyone could understand, who better than a sixth-grade science teacher to help explain the intricacies? Or so we thought. The reviews of Plague include one from a doctor who said the science was “too complex.” As this is such a critical topic in human heath, I want to make it as simple as possible so that everyone can understand.
What are retroviruses?
Retroviruses are classified in a group of RNA viruses called RNA tumor viruses. They are called “retro” because they only have an RNA genome and function differently than other viruses. In most viruses, DNA is transcribed (or written) into RNA, then RNA is translated into protein. Retroviruses, on the other hand, work differently. A retrovirus works by reverse transcribing, that is “writing backwards” into DNA by using an enzyme only retroviruses encode called, “Reverse Transcriptase” (RT). The DNA form of the virus is called a provirus. The provirus is then inserted into the DNA of the host using another enzyme encoded exclusively by retroviruses called “Integrase”. (IN). Integrase cuts open the DNA and then pastes the provirus into the cellular DNA where the provirus lives for the life of the cell.
In addition to RT and IN, retroviruses encode a few other key genes important to make a virus particle called a virion. The envelope gene called env and gag encode the proteins that form an envelope and capsid, which surrounds the RNA genome. The RNA genomes of retroviruses are between seven and twelve thousand bases (7-12 kilobases, kb). The human genome contains approximately three billion base pairs. (RNA is single stranded, while DNA is double stranded, hence “base pairs.”)
A retrovirus virion is approximately 100 nanometers (nM) in size and can only be seen by an electron microscope. The electron micrograph (EM) of the gamma retrovirus we isolated from human blood in 2009 is shown below:

Importantly, the provirus cannot be made into an infectious viral particle without using the machinery of a dividing cell. This is illustrated in the dark parts of the membrane of the cell where the virus is budding out of the cell taking the lipids from the cell membrane to complete the virion.
Here, there, and everywhere
Essentially, all animals have retroviruses integrated in their genomes. Birds, monkeys, cows, pigs, cats, dogs, mice and fish all have retroviruses encoded in their genomes; even plants have retroviruses. Vertebrate genomes harbor thousands of endogenous retrovirus (ERV) elements that display a structure close to that of the integrated proviral form of exogenous retroviruses (gag-, pol-, and env-related regions flanked by 2 LTRs) but the genes are mutated so that they are thought not to be able to produce and release infectious particles. That is, ERVs most likely are the remnants of past infections of the germline by ancestral retroviruses which have been crippled by the immune system of the host. This means that the retroviral genes are defective and no longer release infectious particles. As much as 15% of the human genome is made up of ERV human retroviruses.
In animals, exogenous retroviruses are responsible for some of the deadliest diseases known. Yet, it wasn’t until 1980 when Poiesz and Ruscetti isolated the first human disease-causing retrovirus, then called Human T-cell Leukemia Virus as it was shown to cause an aggressive cancer called Adult T-cell leukemia (ATL). In fact, when my mentor and colleague of 35 years, Frank Ruscetti, joined the National Cancer Institute (NCI) in 1975 to study human disease causing exogenous retroviruses, he was told by NCI scientist John M. Coffin not to bother as they did not exist.
Although retroviruses have been an important part of human evolution as the placenta evolved from ancestral retroviral envelope genes 25-40 million years ago, envelope genes from both exogenous and endogenous retroviruses, aberrantly expressed in humans, have been shown to be responsible for the development of many chronic diseases. The incidence rates of these diseases are skyrocketing in 21st century America and include prostate cancer, breast cancer, leukemia lymphoma, multiple sclerosis, and amyotropic lateral sclerosis (Lou Gherig’s disease).
Expression and mode of development
Many factors are important in the development of diseases associated with retroviruses. The expression and mode of transmission are keys to disease development. We have learned a great deal about the types of diseases from 40 years of study of the mechanisms of disease development from animal and human retroviruses. The two main modes of retrovirus transmission are shown schematically below:
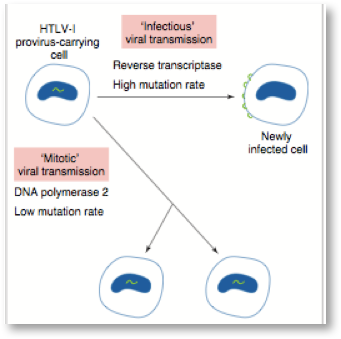
In mitotic transmission, the provirus is dormant or defective and the integrated proviral form of exogenous retroviruses (gag-, pol-, and env-related regions flanked by 2 LTRs) are not expressed. In this case only the daughter cells carry the retroviral genes and if not expressed these endogenous or exogenous retroviral genes remain dormant for years and do not usually contribute to disease until much later in life as the immune system weakens. During infectious transmission, the complete virion is produced with many thousands of virions infecting many neighboring cells and spreading from person to person—both cell free and cell associated—via blood and body fluids. Infectious transmission of HIV drove the AIDS epidemic of the 80s and 90s including transmission from infected cells in a contaminated blood supply and the activation of dormant retroviruses by heavy metals, co-infections and inappropriate vaccination of HIV infected individuals.
Xenograft approaches commonly used since the 1950s in studies of human cancer, autoimmune, and neuroimmune disease promote the evolution of novel retroviruses with pathogenic properties. We now appreciate that it is the use of xenograft technologies in the development of vaccines and biological drugs and genetically modified organisms (GMOs) that have accelerated the spread of animal retroviruses into humans, a process known as zoonosis, whereby an animal retrovirus jumps species, learning to evade immune mechanisms of humans and thereby causing disease.
Read more at: WorldMercuryProject.org
Tagged Under: chronic inflammatory diseases, disease prevention, health, Human Genome, immune system, Retroviruses